Medical Devices
A certificate of compliance with ISO-13485 (Medical Devices – Quality Management Systems – Requirements... View more
NCMR – Key Considerations for the Medical Device Industry
-
NCMR – Key Considerations for the Medical Device Industry
What is NCMR?
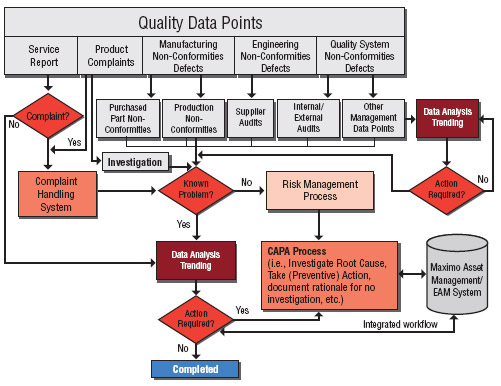
The official definition – Non-Conformance Material Report (NCMR) is a standard way to report non-conforming material that is identified during inspection of items or material (Quality Control or Warehouse Inspection), or during the movement of the materials and/or when the material is in stock.
NCMR is an acronym for Non-conforming material report. When an item does not meet specifications it is identified as discrepant and an NCMR is created. A team meeting is held within the Material Review Board (MRB) to review discrepant items. The MRB consists of: Supplier Quality Engineer (SQE) Manufacturing Engineer (ME).
Information required:
- Details of the source: order, lot identifiers, product…
- Description of the material non-conformance and some category to classify them
- Inspector (who performed the inspection of the material)
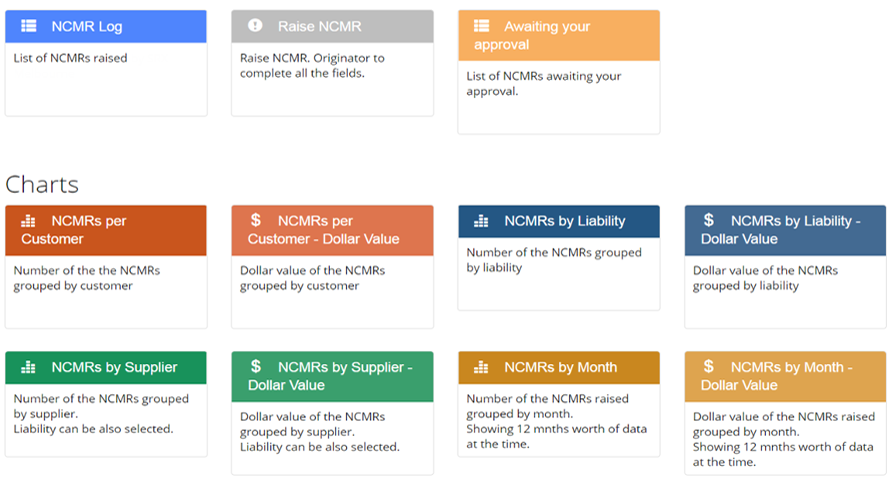
Submit and assign
- The NCMR is submitted for a completeness check, routing decision, and to identify the individual responsible for carrying out the non-conforming material disposition.
- Once it is verified it is assigned to some manager to decide the actions to take.
Disposition of the NCMR
The responsible person/ owner investigates the material non-conformance and can set the NCMR to one of the following proposed dispositions:
- Rework (to Existing Specification): This disposition is for non-conforming material that can be reworked without adverse effect on safety, performance, interchangeability, reliability, or quality. The reworked material is returned to the normal flow of material.
- Rework (to New Specification): This disposition is for non-conforming material that can be reworked without adverse effect on safety, performance, interchangeability, reliability, or quality. Assigns a new part number.
- Reclassify: This disposition involves the reclassification of non-conforming material and the assignment of a new part number.
- Return to Vendor: This disposition is applicable when the material discrepancy is the responsibility of the supplier and other dispositions are not recommended.
- Scrap: The non-conforming material cannot be used for its intended purposes and cannot be repaired.
- Use as is: For items, parts or products for which minor nonconformance is repeated, which can be ignored.
- Repair: This disposition is to bring the nonconforming material to an acceptable condition but which may not totally conform to the applicable drawings or specifications.
- No Fault Found: This disposition is used when the non-conformance is reported erroneously. Material of this disposition is returned to the normal flow.
An NCMR is a Non-Conforming Material Report and it would be opened anytime a material related non-conformance is found. This could be during receiving inspections of raw materials and components, on the manufacturing line, during in-process inspections or final inspection. The report would include a description of the non-conformance, bounding information (this means how much material is affected), and an escalation decision. The escalation decision is generally made by the MRB after reviewing the other information. An NCMR can be escalated to created a CAPA. If the non-conforming material is received from a supplier it might be escalated to a SCAR (Supplier Corrective Action Request). In the case of this simulation a Supplier Corrective Action Request could have been issued except that this supplier was completely unresponsive and not familiar with the requirements of the medical device industry.
Purpose of NCMR:
1. Strong quality control of non-conforming materials reduces the risk of receiving a product that is not compliant or that fails to meet quality expectations.
2. It is the policy to ensure the procedure is in place to identify: segregate and dispose of Non-Conforming Material Report related to safety, legality or quality in a systematic manner and to establish corrective action with the goal of preventing future reoccurrence.
3. The purpose of this procedure is to describe how Non -conforming material, components, partial assemblies & final product are controlled.
In any company ISO 9001 certified, managing non-conforming materials is a must. It is a must not only to satisfy the ISO requirements, but also to reduce costs and ensure product defects are recorded and handled appropriately. When you identify a product or material that does not match the specifications, you record it as a non-conforming material. This action will trigger an assessment, which will determine the type of defect or non-conformance, establish the root cause and decide the disposition for the non-conforming material.
A non-conforming material report (NCMR) is something that must get taken up with the current vendor to prevent the incorporation of defective equipment into a medical device project. An NCMR is generated by either the quality department or during a warehouse inspection and requires identification inputs regarding its condition.
This requires mentioning:
1) The defect at hand and how it negatively impacts the product/project.
2) The lot number that the defective material was manufactured.
3) The identity of the on-site investigator carrying out the examination. Once the NCMR is submitted, a course of action is taken to address the issue, which includes refunding the material to the vendor, scrapping the material to prevent its further use, use it as is and accept its risk, reclassify the material and rework it to another project/product, or repair it so that it is compliant with manufacturing standards.
Sorry, there were no replies found.