Medical Devices
A certificate of compliance with ISO-13485 (Medical Devices – Quality Management Systems – Requirements... View more
FDA Guidance on IEC 62304 Software Standard
-
FDA Guidance on IEC 62304 Software Standard
The international standard IEC 62304 software life cycle processes is a standard which specifies life cycle requirements for the development of medical software and software within medical devices. It is harmonized by the European Union (EU) and the United States (US), and therefore can be used as a benchmark to comply with regulatory requirements from both these markets.
The IEC 62304 standard calls out certain cautions on using software, particularly SOUP (software of unknown pedigree or provenance). The standard spells out a risk-based decision model on when the use of SOUP is acceptable, and defines testing requirements for SOUP to support a rationale on why such software should be used.
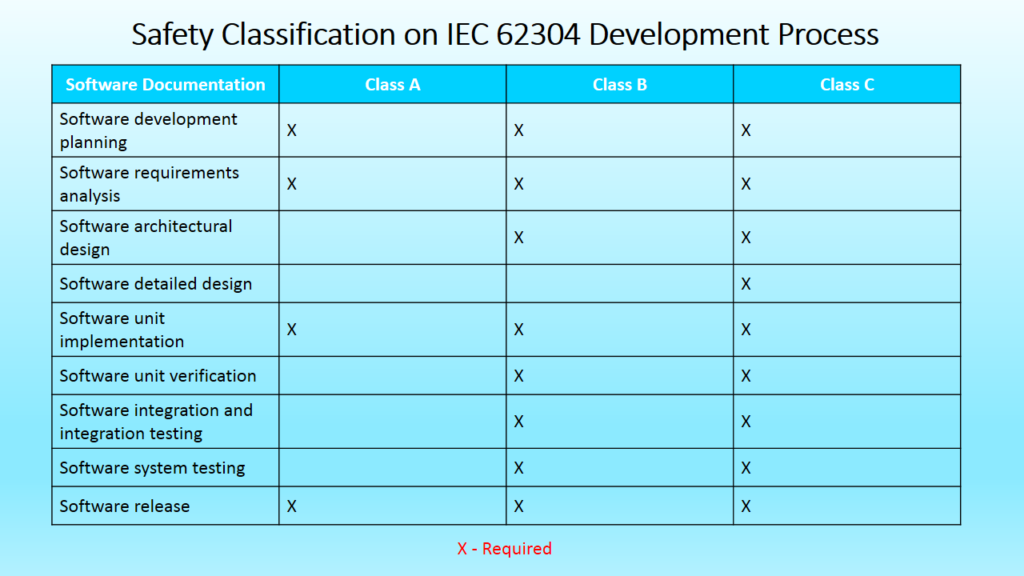
Effect of safety classification on required development process documentation
The IEC 62304 standard provides a framework of software development life cycle processes with activities and tasks necessary for the safe design and maintenance of medical device software. The processes, activities, and tasks described in clause 5 establish a common framework for medical device software life cycle processes that can be understood and shared within and between teams working on a project
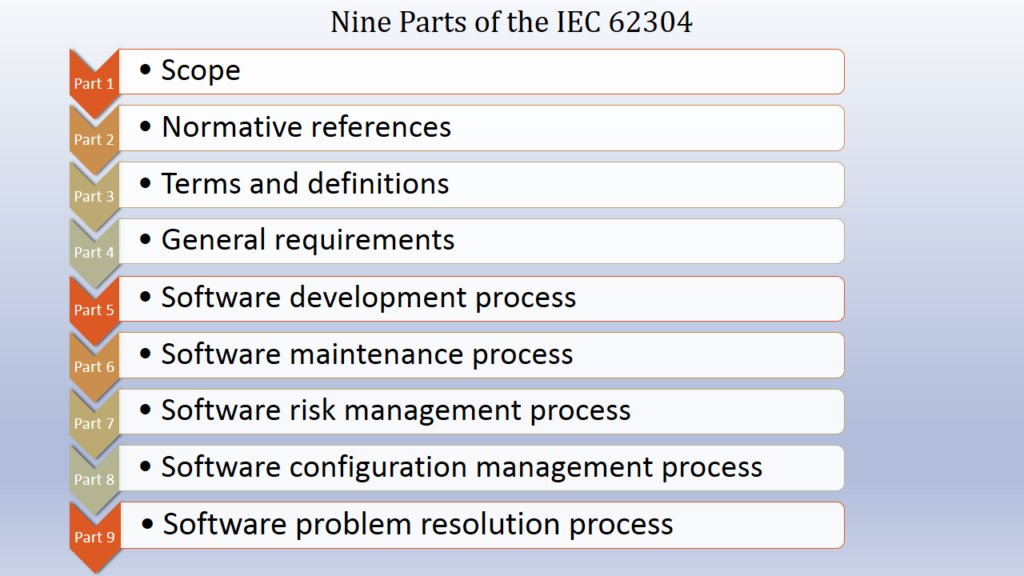
Parts of IEC 62304
IEC 62304 applies to medical device development when software is an integral component to medical device production. It defines the software lifecycle when the software stands alone as a medical device (commonly referred to as software as a medical device, or SaMD), is a component of a medical device, or is used in the production of a medical device.
Relevant FDA Guidance and/or Supportive Publications
1. Guidance for Industry and FDA Staff: Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices.
2. Guidance for Industry, FDA Reviewers and Compliance on Off-the-Shelf Software Use in Medical Devices.
3. General Principles of Software Validation; Final Guidance for Industry and FDA Staff.
4. Guidance for Industry: Cyber-security for Networked Medical Devices Containing Off-the Shelf (OTS) Software.
5. Content of Premarket Submissions for Management of Cyber-security in Medical Devices: Guidance for Industry and Food and Drug Administration Staff.
Sorry, there were no replies found.