Medical Devices
A certificate of compliance with ISO-13485 (Medical Devices – Quality Management Systems – Requirements... View more
IEC 60601 – Medical Design Standards For Power Supplies
-
IEC 60601 – Medical Design Standards For Power Supplies
IEC 60601 is a series of technical standards for the safety and effectiveness of medical electrical equipment, published by the International Electrotechnical Commission. First published in 1977 and regularly updated and restructured, as of 2020 it consists of a general standard, about 10 collateral standards, and about 80 particular standards.
What is the General Standard for IEC 60601?
General requirements for basic safety and essential performance gives general requirements of the series of standards. 60601 is a widely accepted benchmark for medical electrical equipment and compliance with IEC 60601-1 has become a requirement for the commercialization of electrical medical equipment in many countries.
Many companies view compliance with IEC 60601-1 as a requirement for most markets. This standard does not assure effectiveness of a medical device. In the US, evidence of effectiveness is required by the FDA and confirmed through either a Premarket Approval (PMA) or similarity to a predicate device via a 510(k) Premarket Notification.
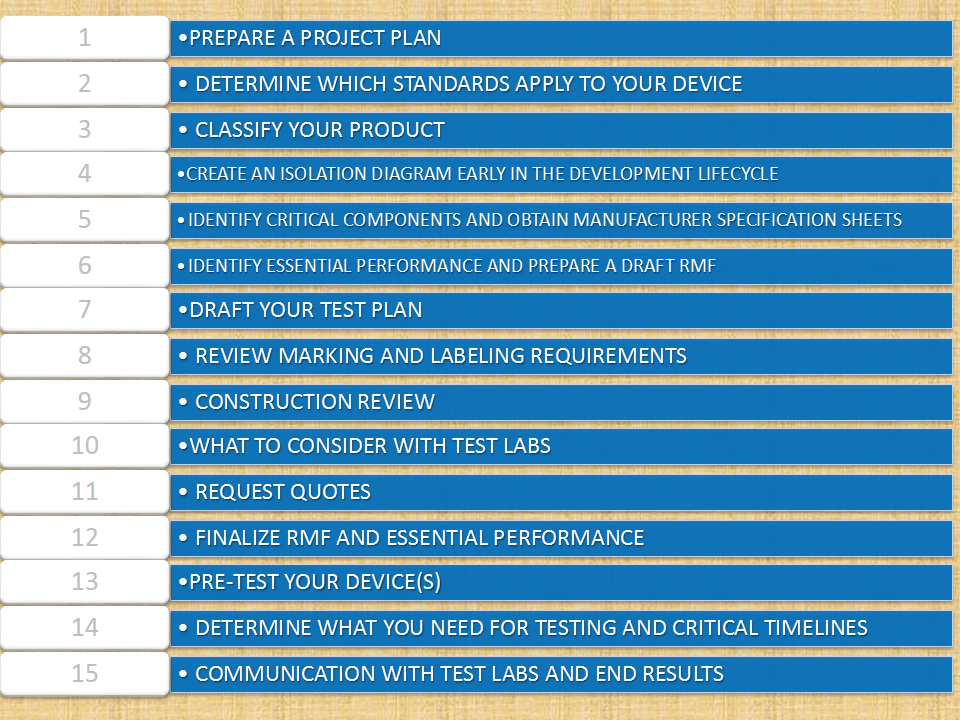
Steps to Getting Approval For IEC 60601-1IEC 60601 StandardsThe Primary Standard
It is referred to as IEC 60601-1 or just “60601,” and compliance with this standard has become a de facto requirement for bringing new medical devices to market in many countries. There are European (EN 60601-1) and Canadian (CSA 60601- 1) versions of the standard that are identical to the IEC standard. There are also deviations from the standard that relates to country-specific requirements.
Collateral Standards
Within IEC 60601-1, there are “collateral” standards that are denoted as IEC 60601-1-x; for example, IEC 60601-1-2 is the EMC collateral standard. Other collateral standards include 60601-1-3, covering radiation protection for diagnostic x-ray systems, 60601-1-9 relating to environmental design, and 60601-1-11 recently introduced for home healthcare equipment.
Particular Standards
As well as collateral standards, there are also many “particular” standards, denoted as IEC 60601-2-x that define specific requirements related to particular types of products, e.g. 60601-2-16 covers blood dialysis and filtration equipment. These particular standards are largely outside the scope of this paper.
Sorry, there were no replies found.