Medical Devices
A certificate of compliance with ISO-13485 (Medical Devices – Quality Management Systems – Requirements... View more
Biological Evaluation of Medical Devices – ISO 10993
-
Biological Evaluation of Medical Devices – ISO 10993
What is ISO 10993?
For nearly 10 years, Technical Committee 194 of the International Organization for Standardization (ISO) and its various working groups have been developing the documents known collectively as ISO 10993, a set of harmonized standards that address the biological evaluation of medical devices. During most of that period, the U.S. device industry has operated according to the Tripartite Guidance for medical device biocompatibility, which was introduced in 1987.
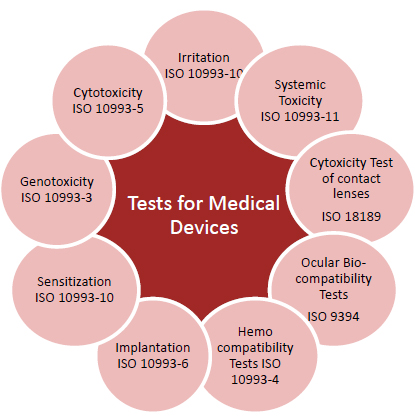
THE ISO 10993 STANDARDS
Technical Committee 194 meets annually in the spring to review progress made on the various 10993 standards and to chart a course for the coming year. Recent meetings were held in Stockholm in 1996 and in the UK city of York in 1997; the 1998 meeting will be held in the Washington, DC area. To date, 12 ISO 10993 standards have been issued by the committee:
- 10993-1: “Guidance on Selection of Tests.”
- 10993-2: “Animal Welfare Requirements.”
- 10993-3: “Tests for Genotoxicity, Carcinogenicity, and Reproductive Toxicity.”
- 10993-4: “Selection of Tests for Interactions with Blood.”
- 10993-5: “Tests for Cytotoxicity—In Vitro Methods.”
- 10993-6: “Tests for Local Effects after Implantation.”
- 10993-7: “Ethylene Oxide Sterilization Residuals.”
- 10993-8: No title assigned.
- 10993-9: “Degradation of Materials Related to Biological Testing.”
- 10993-10: “Tests for Irritation and Sensitization.”
- 10993-11: “Tests for Systemic Toxicity.”
- 10993-12: “Sample Preparation and Reference Materials.”
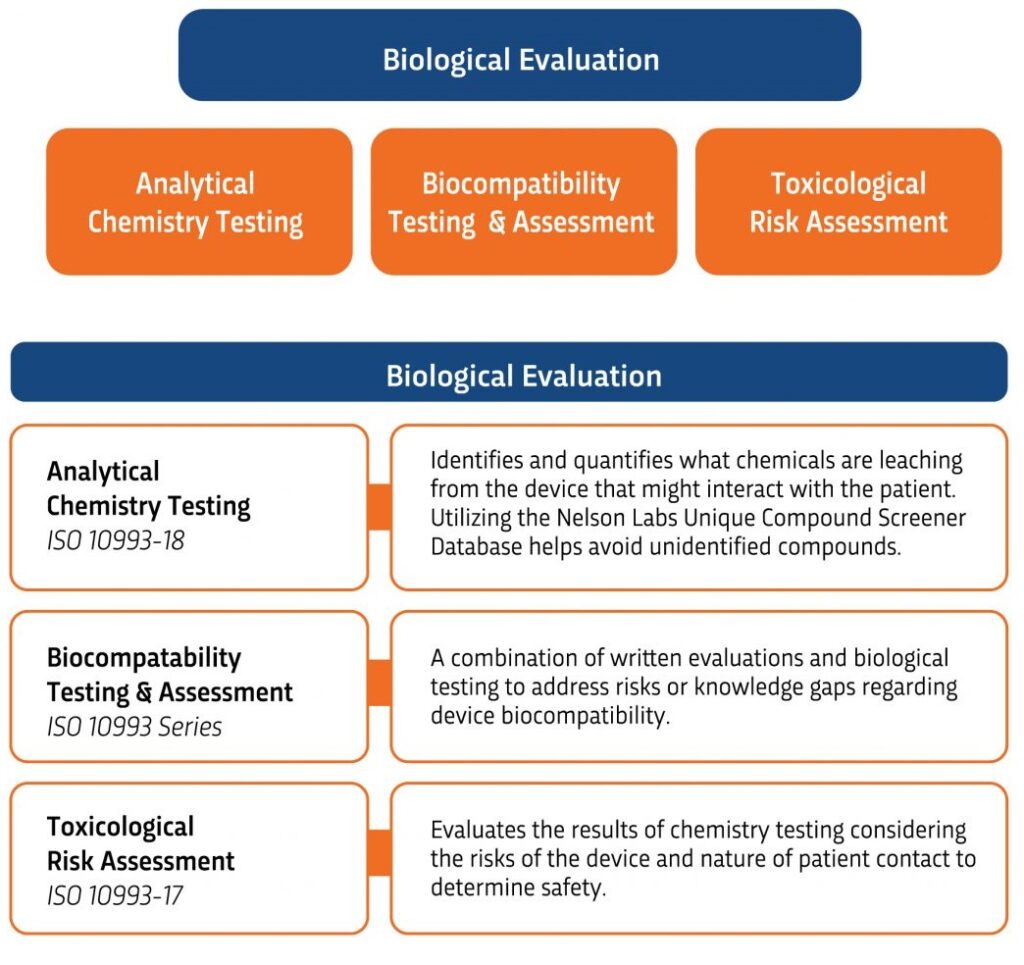
In today’s world, there is continued interest in how an evaluation is conducted long before any biological tests are performed. And, with the release of ISO 10993-1:2018 in August 2018, there is a continued focus in biological safety planning and implementation processes, as well as the characterization of materials. Once again, medical device manufacturers are being asked to go beyond the simple process of identifying a set list of biocompatibility tests when evaluating a device’s biological safety.
Two other standards are currently under review in draft form: ISO 10993-13, “Identification and Quantification of Degradation Products from Polymeric Medical Devices” and ISO 10993-16, “Toxicokinetic Study Design for Degradation Products and Leachables.” In addition, Working Group 14 has written a preliminary draft document dealing with the characterization of materials, a subject that is expected to receive considerable attention in the coming year as it relates to biocompatibility evaluation. Working Group 15, on a strategic approach to biological assessment, was organized to provide overall guidance and advice to TC 194 and to promote internationally uniform application of the standards. Its goal is to minimize the amount of testing that is needed and to optimize the biological safety evaluation of medical devices. This working group is not charged with developing a standard.
Sorry, there were no replies found.